Key Dates
| Cycle 5 Program Announcement and Applications Open | October 6, 2025 |
|---|---|
|
October 20, 2025, 12:00 UTC |
|
| Letter of Intent (LOI) Due Date (online submission) | December 19, 2025, 23:59 UTC |
| Application Due Date | February 6, 2026, 23:59 UTC |
| Scientific Merit Review | March 2026 |
| Decisions Announced | April 10, 2026 |
| Training Start Date | May 11, 2026 |
Training Opportunity Description
Trainees will fall into two groups:
- Principal Investigator Track (PI-STARS) for doctoral-level investigators will be trained to demonstrate leadership in independent and collaborative research. Participants in the PI Track will become proficient in research oversight and governance and build the skills and a scientific project focus toward independent extramural grant funding. PI-STARS will focus on training and their research projects around the following key areas of research interest:
- Fundamental research that provides insight into cancer mechanisms, biomarker discovery, and translation of basic/biomarker information for etiological or interventional research.
- Epidemiologic studies to understand cancer etiology and risk, including genetic and non-genetic factors.
- Cancer informatics and analytics, data integration, and data utilization (including research using data in the public domain).
- Behavioral or psycho-oncology research.
- Prevention, early detection, and screening modalities.
- Development and implementation of low-cost early detection and disease monitoring technologies and interventions.
- Implementation and dissemination science.
It is expected that the mentored training experience will provide:
- A strong foundation in rigorous research design, methods, and analytic techniques appropriate to the applicant’s research and career goals.
- An enhanced ability to conceptualize and think through research problems with increasing independence;
- Guide research using appropriate, state-of-the-art methods.
- The opportunity to present and publish research findings (including first authorship as applicable) and to interact with members of the scientific and medical communities at professional meetings and workshops.
- Professional and scientific skills needed to transition to the next stage of the candidate’s research career; and
- Refinement of the candidate’s understanding of the health-related sciences and the relationship of his/her research to health and disease.
This training opportunity is designed specifically for candidates proposing research that does not involve undertaking an independent clinical trial or an ancillary clinical trial but does allow candidates to propose research experience in a clinical trial led by a sponsor or co-sponsor. Feasibility studies leading to improved clinical trial operations and research that may lead to clinical trials in the future are within the scope of STARS training. Applications are required to affiliate with one or more institutions on our approved STARS institutions and incorporate exceptional mentorship for their projects.
Training Period
- Month 1-2: PROMOTE Africa Core Curriculum
- Months 3-9: Project development and implementation, seminars, mentor interactions, and ancillary targeted course participation.
Applicant Eligibility
Eligible Organizations must be located in Sub-Saharan Africa, and include:
- Higher Education Institutions including: Public/State Controlled Institutions of Higher Education and Private Institutions of Higher Education
- Nonprofit Organizations Other Than Institutions of Higher Education, including research institutes or centers.
- Governments: Eligible Agencies of Local, State, Provincial, or Federal Government
- Clinical Service Providers: Hospitals, Medical Centers and other Free-standing Clinical Service Providers
Employees of For-Profit Organizations are not eligible to apply.
Any candidate with the skills, knowledge, and resources necessary to carry out the proposed research as a PI is invited to work with his/her mentors and organization to develop an application for support. African citizens resident in a Sub-Saharan African Country are eligible to apply. Applicants can be affiliated with one of the Approved STARS institutions or at another African institution if the facilities and resources for training can be adequately justified.
Successful candidates will demonstrate evidence of high academic performance in relevant sciences and commitment to a research career. PI-STARS applicants should have obtained a Ph.D., MD, DO, DC, DDS, DVM, OD, DPM, ScD, EngD, DrPH, DNSc, MBBS, MBBCh, ND (Doctor of Naturopathy), PharmD, DSW, PsyD, or equivalent doctoral degree from an accredited institution. Certification by an authorized official of the degree-granting institution that all degree requirements have been met is also acceptable. PI-STARS training cannot be used to support the clinical years of residency training. However, these awards are appropriate for the research fellowship years of a residency or fellowship program.
Eligibility: PM-STARS
Any candidate with the skills, knowledge, and resources necessary to carry out the proposed project management training and project related to PM is invited to work with his/her mentors and organization to develop an application for support. PM-STARS must be an African citizen resident in a Sub-Saharan African Country who will undertake their research projects at one of the Approved STARS institutions. The candidate must show evidence of high performance in relevant clinical or research areas and have a commitment to a career in project management. No specific degree or prior certifications are required to be eligible to apply for PM-STARS. However, it is anticipated that successful PM applicants will have training in nursing, psychology, management, health care, social work, or other related disciplines. Doctoral level trained candidates (e.g., Ph.D., MD, DDS, DVM, MBBS, MBBCh, DSW, PsyD) are also eligible to apply.
Resubmissions
The STARS Program will not accept duplicate applications from the same candidate in the same training cycle. However, applicants who were unsuccessful with an application can resubmit their application in a subsequent training cycle.
Information for Applicants
Applicants should carefully follow all instructions when completing the application form.
Sponsor
Before applying, the candidate must identify a Sponsor who will supervise the proposed mentored research and career development experience. The primary sponsor should be an active investigator in the proposed research training and be committed to the candidate’s research training, direct supervision of his/her research, and career development. The sponsor must document the availability of sufficient research support, facilities, and environment to achieve high-quality research training. The Sponsor must provide a letter of support detailing how they will guide the candidate to complete their program and achieve future career success. Ideally, the sponsor should be appointed and have ongoing research activities at the applicant’s primary training institution. If the trainee is proposing training at a site that is not already a pre-approved site, then it is possible to submit a request to be included by completing a survey providing information demonstrating the capacity for research at the center must be provided.
The Sponsor should describe the roles and responsibilities that both he/she and the trainee are undertaking, including contributions to the research plan, the portion of the training and research ideas and plan that originated with the candidate, and the relationship between the proposed plan and funded or unfunded research projects previously devised by the sponsor.
Mentoring Team
In addition to the primary sponsor, the applicant should identify additional key mentors who will provide support and guidance to relevant aspects of the training and projects. Multiple individuals may be identified to represent specific areas of expertise and must provide a letter of support describing their role in training. It is advised that the mentoring team be no more than four individuals, including the Sponsor. The mentors may be located at institutions other than the applicant’s home institution. PI-STARS applicants are required to identify a mentoring team for their training, but are not required to identify these mentors at the time of application. Mentors can be identified with the support of the training staff and Sponsor at the time of training. A mentoring team is optional but encouraged for PM-STARS applicants.
Applicant Information
Applicants will provide written information (see application form) that address three areas:
- Applicants should describe their prior training and experiences that have led them to seek this training (500 words)
- Applicants should identify the skills, theories, conceptual approaches, etc., to be learned or enhanced during the training. For the PI track, include expertise in rigorous research design, experimental methods, quantitative approaches, and data analysis and interpretation, as applicable. (1 page)
- Applicants should provide a training plan that explains the importance of a problem, need, or a critical barrier to progress that the proposed training addresses. They should describe the strengths and weaknesses in the rigor of prior work (both published and unpublished) that justifies the need for the proposed training and explain how the proposed training will improve scientific knowledge, technical capability, or clinical practice in one or more broad cancer fields. For the PI Track, the applicant can describe how the concepts, methods, technologies, treatments, services, or preventative interventions that drive this field will be changed if the proposed aims are achieved. If the candidate is proposing to gain experience in a clinical trial as part of his or her research training, describe the relationship of the proposed research project to the clinical trial (1 page).
Letters of Support
The applicant should provide two letters of support, one of which should be from the Sponsor.
Submission Requirements and Information
Applications must be submitted electronically. Paper applications will not be accepted. Upon receipt, applications will be evaluated for completeness and compliance with application instructions. Applications that are incomplete or non-compliant will not be reviewed.
Summary of Pre-Application Steps
- Identify a Sponsor Institution (at least 2-3 months before the application deadline).
- Discuss the Sponsor’s role in your training, and establish an agreement about their role in the training (at least 2-3 months before the application deadline)
- Write application statements (at least 2 months before the application deadline, with time for review by Sponsor and Mentor Team members).
- Applicant Statement 1: Candidate training and experience that explains why you seek this training.
- Applicant Statement 2: Statement and justification of the problems/needs that this training will address, and what impact you can have to address these problems/needs.
- Applicant Statement 3: Training plan (including ancillary coursework, meeting attendance, site visits, or exchanges) and project concept (to be fully developed and refined during the training period).
- Develop a training budget and justification (at least one month before the application deadline)-for PI trainees only.
- Complete applicant biosketch.
- Collect biosketches from Sponsor and Mentor Team members
- Submit Application, including Institution, Sponsor, and Mentor Team letters; biosketches, and supporting documentation.
Application Review
Successful STARS applications will have identified a clear motivation and career goals that will be enhanced by the training. In addition, successful PI-STARS applications have a research project that is integrated with the training plan. The review will emphasize the candidate’s potential for a productive career, the candidate’s need for the proposed training, and the degree to which the research project and training plan, the sponsor(s), and the environment will satisfy those needs. The criteria that will be used to evaluate applications are outlined below. These criteria can guide the content of the application, letters of support, and other documentation.
Overall Impact: Reviewers will provide an overall impact score to reflect their assessment of the likelihood that the fellowship will enhance the candidate’s potential for, and commitment to, a productive, independent scientific research career in a health-related field, in consideration of the scored and additional review criteria.
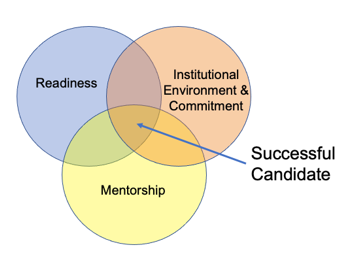 |
The successful STARS applicant will be
|
|---|
Applicant:
- Are the candidate’s academic record and prior experience of high quality?
- Does the candidate have the potential to develop into an independent and productive PI or PM?
- Does the candidate demonstrate commitment to a research career in the future?
- Does the project reflect a significant contribution of the candidate to the originality of the project idea, approach, and hypotheses relative to the candidate's career stage?
Sponsors and Mentoring Team:
- Is the sponsor(s’)/Mentor(s’) research qualifications (including recent publications) and track record of mentoring individuals at a similar stage appropriate for the needs of the candidate?
- Is there evidence of a match between the research and clinical interests (if applicable) of the candidate and the sponsor(s)/Mentor(s)?
- Do(es) the sponsor(s)/Mentor(s) demonstrate an understanding of the candidate’s training needs as well as the ability and commitment to assist in meeting these needs?
- Is there evidence of adequate research funds and infrastructure to support the candidate’s proposed research project and training for the duration of the research component of the fellowship?
- Are the qualifications of Mentor Team members, including their complementary expertise and previous experience in fostering the training of fellows, appropriate for the proposed training?
- Does the sponsor’s/mentors’ research and training record, as well as mentoring statement, indicate that the candidate will receive outstanding training in the proposed research area and have the opportunity to publish high-quality papers and present research data at national meetings as the project progresses (PI-STARS) or will lead to a career that can achieve success in project management (PM-STARS)?
PI-STARS Research Project:
- Is the proposed research project of high quality, and is it well integrated with the proposed research training?
- Is the prior research that serves as the key support for the proposed project rigorous?
- Has the candidate included plans to address weaknesses in the rigor of prior research that serves as the key support for the proposed project?
- Has the candidate presented strategies to ensure a robust and unbiased approach appropriate for the work proposed?
- Based on the sponsor’s description, is the candidate’s proposed project sufficiently distinct from the sponsor’s ongoing funded research for the candidate’s career stage?
- Is the project consistent with the candidate’s stage of research development?
- Is the proposed time frame feasible to accomplish the proposed training and project?
- Does the training plan provide adequate opportunities to present and publish research findings and meet with scientists in the community at national meetings as the work progresses?
- Will the training plan provide the professional skills needed for the candidate to transition to the next stage of his/her research career?
- If proposed, will the clinical trial experience contribute to the proposed project and the candidate’s research training?
Future Potential:
- Are the proposed research project and training plan likely to provide the candidate with the requisite individualized and mentored experiences to obtain appropriate skills for a research career?
- Does the training plan take advantage of the candidate’s strengths and address gaps in needed skills?
- Does the training plan document a clear need for, and value of, the proposed training?
- Does the proposed training have the potential to serve as a sound foundation that will enhance the candidate’s ability to develop into a productive researcher?
Institutional Environment & Commitment to Training:
- Are the facilities, resources (e.g., equipment, laboratory space, computer time, subject populations, clinical training settings), and training opportunities (e.g., seminars, workshops, professional development opportunities) adequate and appropriate?
- Is the institutional environment for the candidate’s scientific and clinical development of high quality?
- Is there an appropriate institutional commitment to fostering the candidate’s mentoring training?
- Does the institutional and lab environment provide appropriate and sufficient opportunities for the candidate to gain the professional skills needed for a successful research career?
Ethical and Safety Considerations
The applicant should discuss any issues related to research ethics and compliance. The applicant need not obtain approval to undertake research in the application, but ethics approvals will be required before any research is conducted during this program. The discussion of anticipated ethical considerations should include:
Inclusion of Women, Minorities, and Individuals Across the Lifespan: When the proposed project involves human subjects and clinical research, the review will evaluate the proposed plans for the inclusion (or exclusion) of individuals based on sex/gender, race, and ethnicity, as well as the inclusion (or exclusion) of individuals of all ages (including children and older adults) to determine if it is justified in terms of the scientific goals and research strategy proposed.
Vertebrate Animals: The committee will evaluate the involvement of live vertebrate animals as part of the scientific assessment according to the following criteria: (1) description of proposed procedures involving animals, including species, strains, ages, sex, and total number to be used; (2) justifications for the use of animals versus alternative models and the appropriateness of the species proposed; (3) interventions to minimize discomfort, distress, pain and injury; and (4) justification for euthanasia method if NOT consistent with the AVMA Guidelines for the Euthanasia of Animals. Research may not involve the use of non-human primates.
Biohazards: Reviewers will assess whether materials or procedures proposed are potentially hazardous to research personnel and the environment, and if needed, determine whether adequate protection is proposed.
Training in the Responsible Conduct of Research
All accepted STARS trainees will participate in Responsible Conduct of Research (RCR) training.
Review and Selection Process
Applications will be evaluated for scientific and technical merit by a committee of experts using the criteria described above. As part of the review process, applications may undergo a committee process. Only those applications deemed to have the highest scientific and technical merit (generally the top half of applications under review) will be discussed and assigned an overall impact score. All applications will receive a written critique. The following will be considered in making funding decisions:
- Scientific and technical merit of the proposed project as determined by scientific peer review.
- Availability of funds.
- Relevance of the proposed project to program priorities.
- Diversity of trainees, including gender, geography, and discipline.
Inventions and Copyrights
STARS training is funded primarily for educational purposes. All discoveries, inventions, and publications are the property of the trainees and their Sponsors and Mentor Teams and their affiliated institutions. STARS has no claim on research output generated under this training or associated research projects.
Reporting
After the training period, trainees will be required to submit the Research Performance Progress Report (RPPR). The report is due two months after the end date of the training period and must include information describing the trainee’s progress and future research and training plans.
In carrying out its stewardship of training programs, STARS may request information essential to assessing the effectiveness of this program from databases and participants themselves. Participants may be contacted after the completion of this award for periodic updates on various aspects of their employment history, publications, support from research grants or contracts, honors and awards, professional activities, and other information helpful in evaluating the program's impact.
Contact Information
We encourage inquiries concerning this funding opportunity and welcome the opportunity to answer questions from potential applicants. Please contact us at d43grant@dfci.harvard.edu




